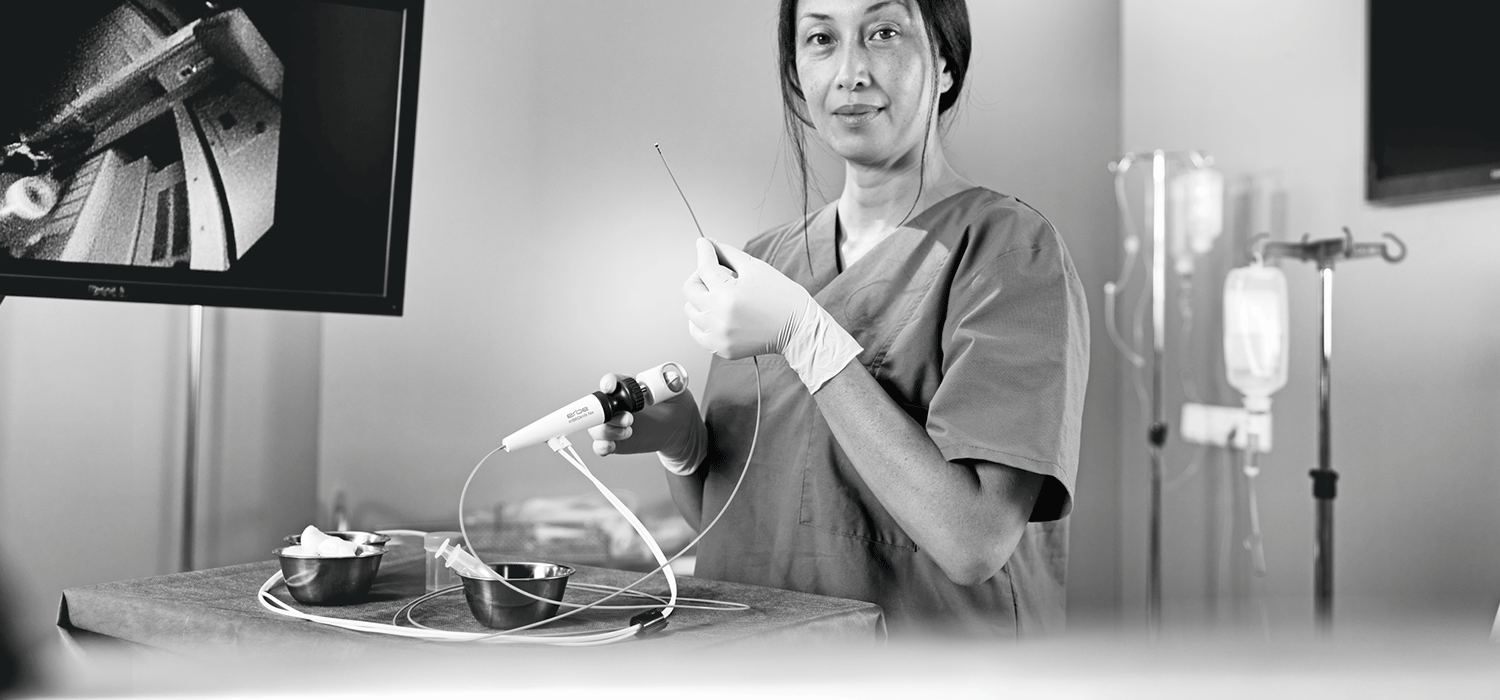
Erbe Elektromedizin introduces innovating instrument HYBRIDknife® flex: Now CE marked
An enhanced instrument for colorectal ESD, successfully adopted in initial human cases.
Tübingen, Germany - Erbe Elektromedizin GmbH, a global leader in medical technology, is proud to announce the first clinical adoption of HYBRIDknife® flex, the latest addition to their portfolio of hydrodissection and electrosurgical devices. The company is honored to have been guided by therapeutic and surgical endoscopists worldwide in realizing this newest addition to lesion management within the gastrointestinal tract.
In the initial human cases, the endoscopy team led by Prof. Alessandro Repici at Humanitas Research Hospital in Milan, Italy, successfully removed a 50x65 mm lateral spreading tumor in the cecum and a 35x40 mm lateral spreading tumor in the colon. Both lesions were removed en bloc without complications.
The procedures took place at Humanitas Research Hospital in Milan. Prof. Alessandro Repici and Prof. Roberta Maselli performed the procedures.
“This is an historical time for us as endoscopists. A new generation of knifes have been developed by the Erbe team and in the last few days we had the privilege of using them for the first time in human cases. We wish to acknowledge the entire Erbe team, who did a great job in completely reengineering the device with new features leading to benefits. The new HYBRIDknife flex is an advanced multifunctional device that combines Erbe waterjet technology with new precise functions and features specifically designed for colorectal ESD. This unique device will definitely make ESD procedures easier, faster and safer. Congratulations to Erbe for delivering such outstanding technology, which is going to be a true game changer for ESD and third space procedures.”
Prof. Alessandro Repici,
Chairman of Department of Gastroenterology and Hepatology,
Humanitas Research Hospital, Milan
HYBRIDknife® flex incorporates the inventive approach of needle-free, high-pressure hydrodissection and novel electrosurgical waveforms within the same device. The proprietary high-pressure injection provides more consistent and reliable separation and expansion of the submucosal tissue layer versus standard injection, even in challenging anatomy, e.g., over folds or in the presence of submucosal fibrosis, and with no need for instrument exchange. The extended electrode maintains a stable length even in difficult angled or retroflexed endoscopic positions. The thin electrode of the HYBRIDknife® flex leads to precise cutting with limited thermal damage.
The HYBRIDknife® flex will be available in I and T-types, both of which come in two different electrode lengths.
- T-type 1.5 mm short
- T-type 2.0 mm long
- I-type 1.5 mm short
- I-type 2.0 mm long
With the HYBRIDknife® flex, physicians can perform all four essential steps of an ESD without changing the instrument. The knife allows for the marking of lesions, layer-specific elevation of the submucosa using a high-pressure waterjet for a needle-free injection, incision, and dissection with electrosurgical modes such as endoCUT® and preciseSECT®, and if required, coagulation.
The HYBRIDknife® flex recently received the CE marking, and Erbe has submitted an application to the U.S. Food and Drug Administration (FDA) for regulatory approval, which will expand the reach of this innovative instrument to healthcare professionals around the world.
Erbe is humbled by the direction and collaboration from physicians worldwide in realizing this important combination device. They are honored to ultimately serve patients through the expertise of doctors, nurses, technicians, and biomedical engineers.
“We are very excited to announce the first in human cases with our latest addition to the Erbe HYBRIDknife® portfolio. An innovative instrument enabling high performance in advanced procedures, developed on the well-known foundation of a single-device approach to ESD and needle-free injection, to address the safety concern of advanced interventional procedures in the gastrointestinal tract. It is an honor to have partners around the world who accompanied us throughout the entire product development process. We expect HYBRIDknife® flex to be commercially available in 2024.”
Mariuccia Zambelli,
Vice President Global Marketing, Erbe Elektromedizin GmbH
For more information about the Erbe Group, please visit www.erbe-med.com or follow on LinkedIn.
Press release distributed by Pressat on behalf of Erbe Elektromedizin GmbH, on Wednesday 2 August, 2023. For more information subscribe and follow https://pressat.co.uk/
Endoscopy ESD Resection Lesion Management Colorectal En Bloc r0 Needle-Free Water Injection ESD Hybridknife Dissection Thin-Walled Structur Health Medical & Pharmaceutical
Published By

+4970717550
marketing@erbe-med.com
https://www.erbe-med.com
Thomas Hämmerle
Visit Newsroom
You just read:
Erbe Elektromedizin introduces innovating instrument HYBRIDknife® flex: Now CE marked
News from this source: